White Pupae for Green Fields: How CRISPR Is Transforming Fruit Fly Control
17 Nov 2025
In their new article “CRISPR/Cas9-based white pupae mutant lines in Bactrocera spp. for sterile insect technique applications”, published in Insect Science (2025), Chrysanthi Ioannidou, Maria‑Eleni Gregoriou, Marc F. Schetelig, Elena Drosopoulou, Kostas D. Mathiopoulos, and Kostas Bourtzis address the need for reliable, early-stage sex separation across multiple pest species for SIT. The authors present a modern solution by using CRISPR/Cas9 to disrupt the white pupae (wp) gene, producing visible, stable white-pupae mutants in three major Bactrocera pests (B. dorsalis, B. correcta, B. oleae).
Invasive fruit flies of the Bactrocera genus are a major threat to global agriculture, infesting crops such as mangoes, olives, and papayas, and causing billions in economic losses. One of the most environmentally friendly methods to suppress their populations is the Sterile Insect Technique (SIT), where sterilized male flies are released to outcompete wild males in mating. But SIT becomes significantly more efficient when only males are released. For that, Genetic Sexing Strains (GSS) are key—they enable early-stage separation of males and females.
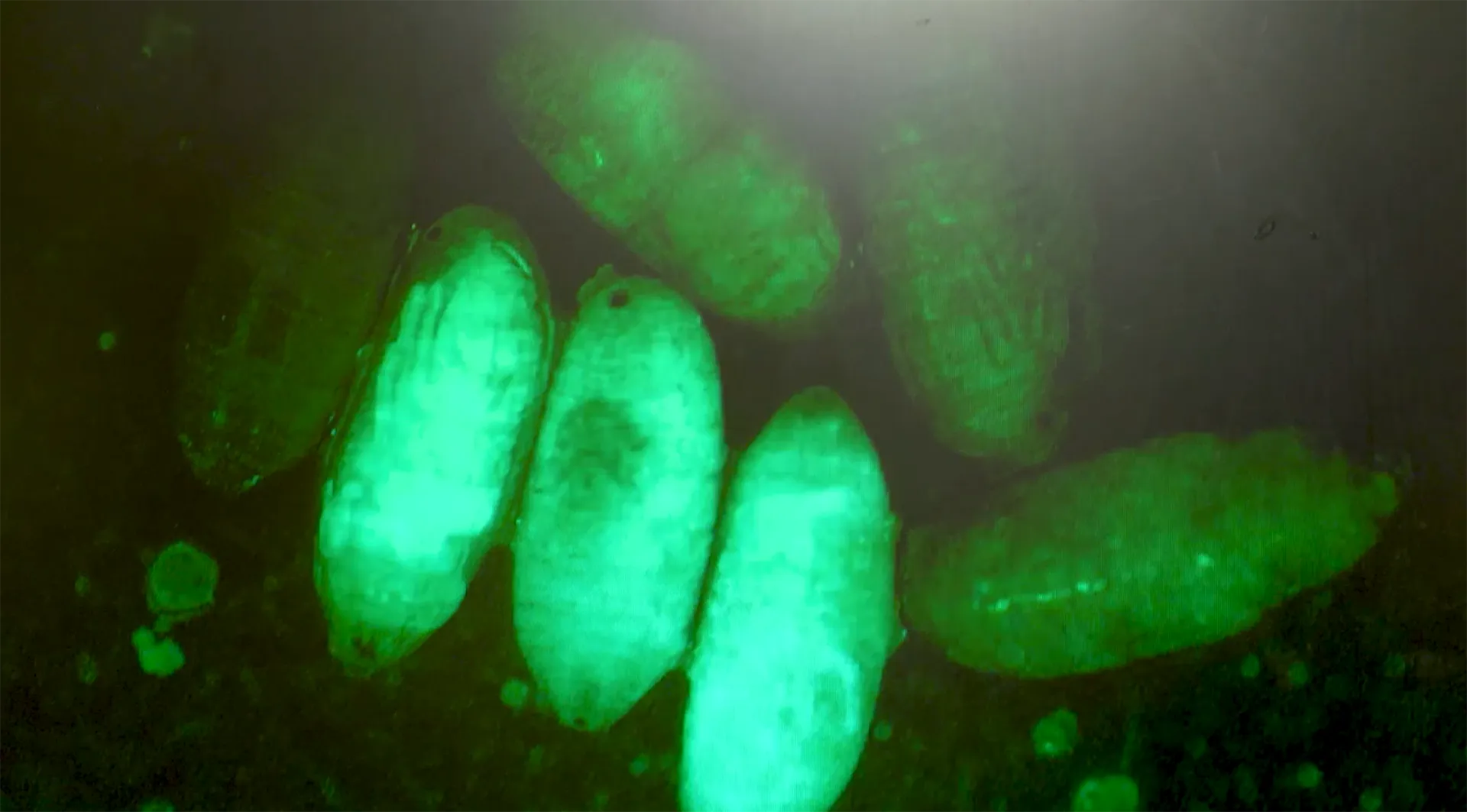
A publication led by Chrysanthi Ioannidou has now taken a big step forward by creating CRISPR-based “white pupae” mutants in three Bactrocera species. By knocking out a single gene (called white pupae or wp), they produced flies with a visible marker: their pupae turn white instead of brown. This allows easy sex identification before the flies even emerge.
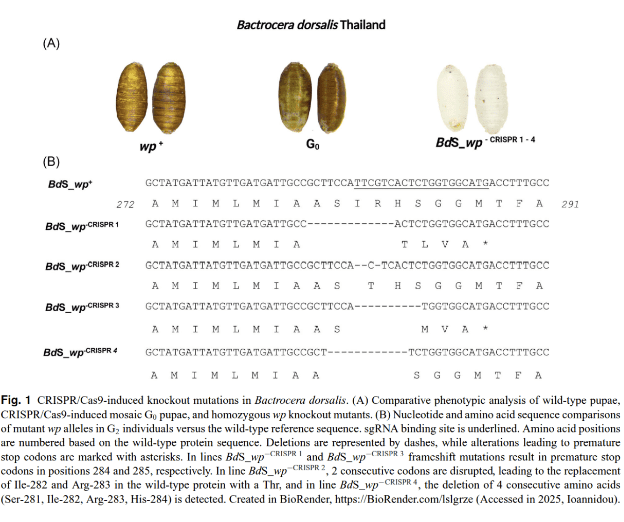
The approach was successfully applied in B. dorsalis, B. correcta, and B. oleae—showing the potential for a cross-species GSS toolkit. It’s a promising step toward scalable, insecticide-free pest control—where gene editing meets agricultural sustainability.
Find the publication on Zenodo: https://zenodo.org/records/17629888